When considering a biologic for a woman with axSpA, PsA, or RA, think CIMZIA®1
CIMZIA® demonstrated long-term broad spectrum efficacy across indications5-22
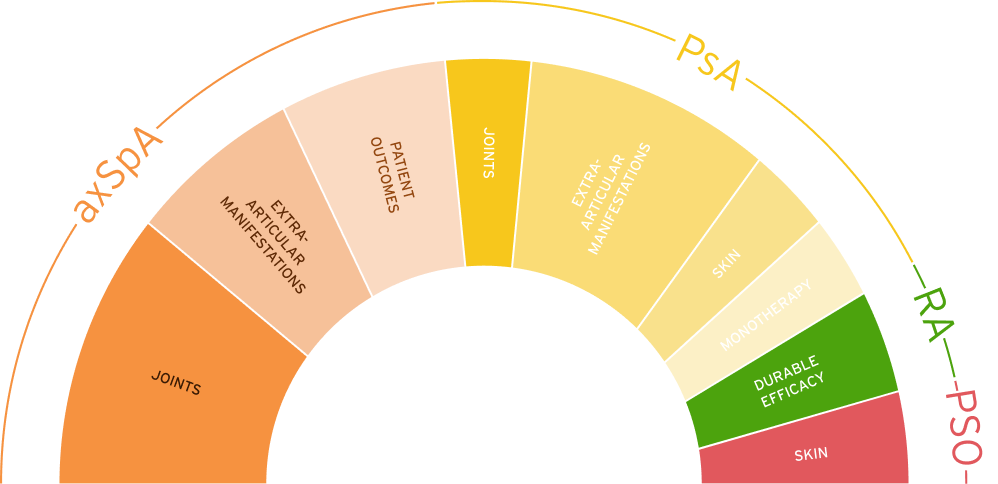
The safety and efficacy of CIMZIA® was assessed in patients with axSpA, PsA, PSO, and RA. In axSpA, CIMZIA® demonstrated efficacy on joints, extra-articular manifestations, and patient outcomes, as shown in the C-OPTIMISEa phase 3b study, RAPID-axSpAb phase 3 study, and C-VIEWc phase 4 study. In PsA, CIMZIA® demonstrated efficacy on joints, extra-articular manifestations, skin, and monotherapy, as shown in the RAPID-PsAd phase 3 study. In RA, CIMZIA® demonstrated durable efficacy in the RAPID 1e, FAST4WARDf, and study 014g phase 3 studies. In PSO, CIMZIA® demonstrated efficacy in the CIMPASI-1h, CIMPASI-2h, and CIMPACT phase 3 studies.5-22
CIMZIA® has a well-characterised, long-term safety profile across all approved indications1,23
Summary of AEs of interest reported for CIMZIA®-treated patients (all doses) in the combined RCT and OLE periods (RCT+OLE)
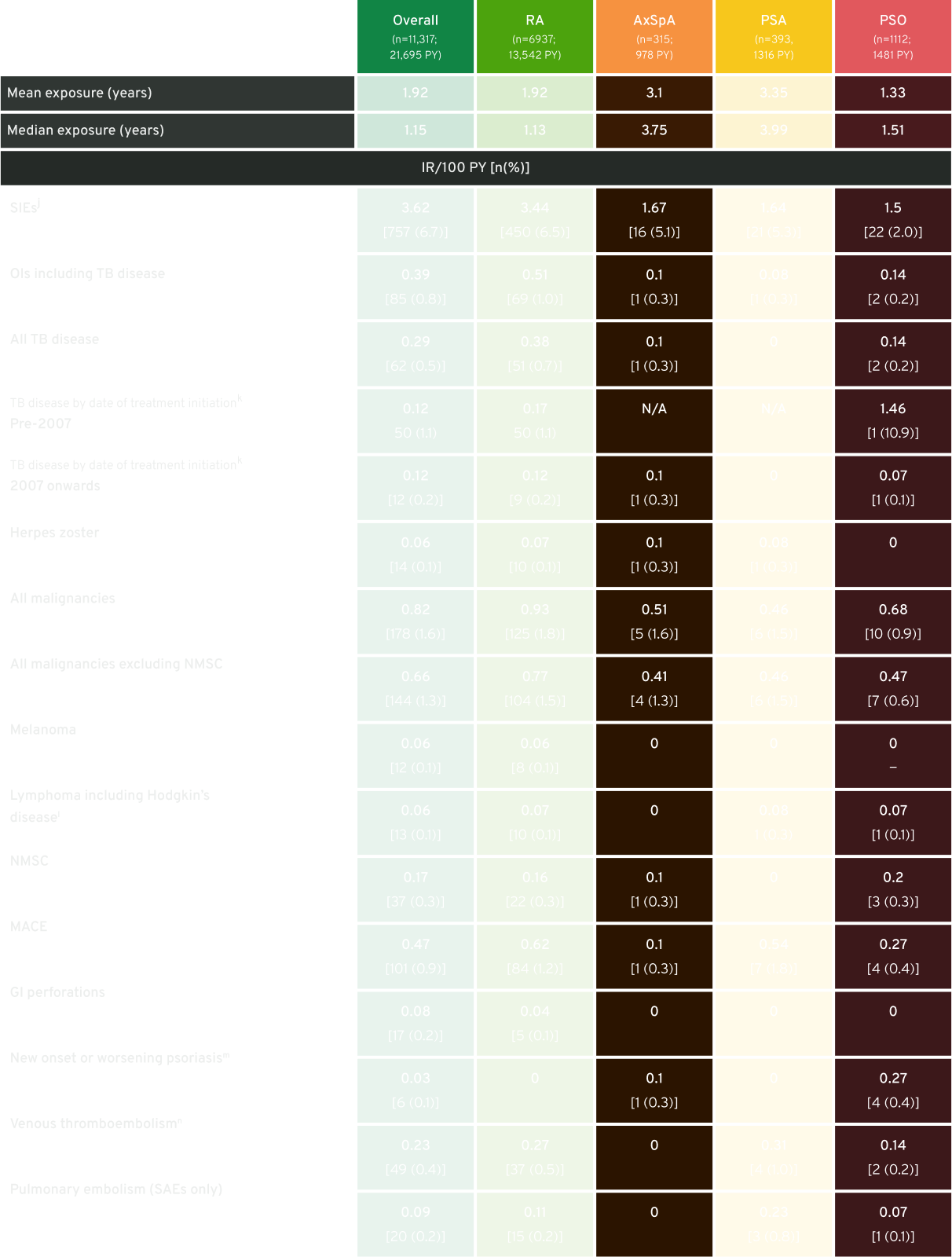
The table shown above is adapted from a pooled analysis of 49 clinical trials that assessed CIMZIA®.23 Please note that not all patients included in these clinical trials received approved CIMZIA® doses.23 The dosing recommendations outlined in the SmPC should always be followed when treating patients with CIMZIA.1
n (%) refers to the number of patients with events; zeros indicate that there were no cases. NMSC includes serious and non-serious cases.
Adapted from Curtis J, et al. 2019.
The use of adequate contraception should be considered for women of childbearing potential. For women planning pregnancy, continued contraception may be considered for 5 months after the last CIMZIA® dose due to its elimination rate, but the need for treatment of the woman should also be taken into account. Data from more than 1,300 prospectively collected pregnancies exposed to CIMZIA® with known pregnancy outcomes, including more than 1,000 pregnancies exposed during the first trimester, does not indicate a malformative effect of CIMZIA®. Further data are being collected as the available clinical experience is still limited to conclude that there is no increased risk associated with CIMZIA® administration during pregnancy. CIMZIA® should only be used during pregnancy if clinically needed. CIMZIA® can be used during breastfeeding.1
Related information
Velit adipisicing adipisicing nostrud irure ullamco sint do veniam in nulla nulla pariatur eu id. Cupidatat mollit adipisicing eiusmod Lorem cupidatat qui est do cillum duis aliqua id do ullamco.
CIMZIA® is not registered for the treatment of uveitis.1
Please refer to the CIMZIA® SmPC for a complete list of licensed indications.1
You may also like…
EU-P-CZ-PsA-2200001
Date of preparation: February 2023



