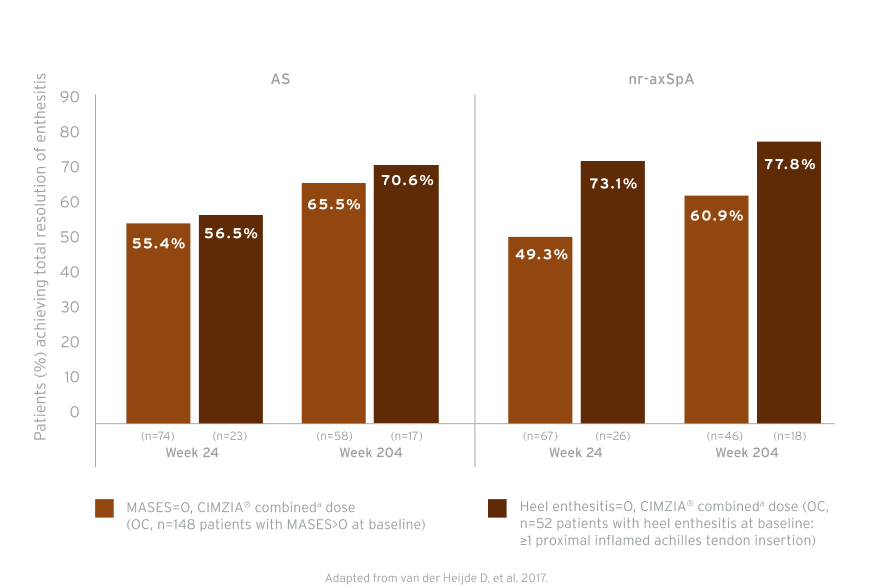
RAPID-axSpA: Total resolution of enthesitis by subpopulations to week 204 (post-hoc analysis)5
CIMZIA® is not registered for the treatment of enthesitis.1
Please refer to CIMZIA® SmPC for a complete list of licensed indications.1
CIMZIA® rapidly reaches target tissues affected by enthesitis in active SpA patients6
Prediction of response to treatment: immunoscintigraphy6
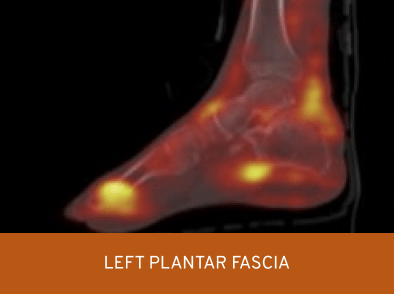

An accumulation of CIMZIA®, 4-5 hours post-injection, is seen in the inflamed tissues of active SpA patients6
Left image shared with UCB by Carron P. Right image adapted from Carron P, et al. 2016. Both images used with the copyright holder’s permission.
SPECT-CT of feet in an active SpA patient with enthesitis of the achilles tendon and left plantar fascia 4-5 hours post-injection with Tc99m radiolabelled certolizumab pegol.6
See the study design section for further information regarding methodology.
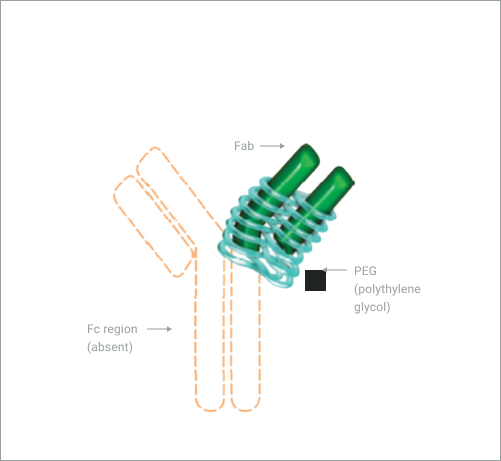
Adapted from Porter C, et a. 2016
CIMZIA® (certolizumab pegol) is the first and only Fc-free PEGylated TNF-α inhibitor1,2,7,8
- PEGylation increases the bioavailability10,11 and delays the metabolism and elimination12 of the drug
- CIMZIA®, due to its unique FC-free structure, showed no to minimal (below 0.1%) placental transferb from mother to baby in almost all casesc in a first-of-its-kind clinical study (CRIB study)2. The clinical significance of low levels of certolizumab pegol for infants is unknown.
CIMZIA® is not registered for the treatment of enthesitis.1
CIMZIA® should only be used during pregnancy if clinically needed.1
Please refer to the CIMZIA® SmPC for a complete list of licensed indications.1
Related information
Velit adipisicing adipisicing nostrud irure ullamco sint do veniam in nulla nulla pariatur eu id. Cupidatat mollit adipisicing eiusmod Lorem cupidatat qui est do cillum duis aliqua id do ullamco.
You may also like…
EU-P-CZ-PsA-2200001
Date of preparation: February 2023



